Aufbau Principle
Understanding how electrons fill atomic orbitals is one of the biggest challenges in learning nuclear structure. That’s where the Aufbau Principle comes in. This fundamental concept in chemistry explains the order in which electrons occupy orbitals. It also builds the foundation to easily grasp more advanced topics later, such as orbital hybridization, quantum numbers, and the behavior of transition metals.
In this lesson, you’ll learn:
• What the Aufbau Principle is and why it matters
• The formula that determines the electron filling order
• How many electrons each type of orbital can hold
• Additional rules (Hund’s Rule and Pauli Exclusion Principle) for writing accurate electron configurations
A Definition of the Aufbau Principle
The Aufbau Principle, derived from the German term "Aufbau" meaning "building up," states that in the ground state of an atom, electrons occupy the lowest-energy atomic orbitals first before they start filling the higher-energy ones. This process follows a specific order determined by the n + ℓ rule, where n represents the principal quantum number and ℓ represents the azimuthal quantum number, ensuring a stable electron configuration.
In simple terms, this fundamental rule helps us understand how electrons occupy an atom's energy levels (or "shells"), starting at the lowest energy level available and then moving up to higher levels. It’s like climbing a staircase from the bottom step up.
Aufbau Principle Formula
The Aufbau Principle formula dictates the order in which electrons are filled in atomic orbitals. The sequence of filling is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p.......
The diagram below illustrates the order in which electrons fill their orbitals.

Electrons fill orbitals in a specific order based on their energy levels, starting with the 1s orbital, then moving to the 2s, followed by the 2p, and so forth. However, as shown in the diagram, the 4s shell is filled before the 3d orbital because it has a lower energy level.
Also, keep in mind that the Aufbau Principle has several exceptions, especially among transition metals and heavier elements. These exceptions occur because atoms sometimes gain increased stability from having half-filled and fully filled orbitals.
Take Chromium (Cr) as an example. Instead of the expected configuration 1s2 2s2 2p6 3s2 3p6 4s2 3d4, the actual configuration is [Ar] 3d⁵ 4s¹. This is because a half-filled 3d subshell (with 5 electrons) is more stable than a partially filled orbital, so one electron from the 4s orbital shifts to the 3d subshell.
Other common exceptions include Copper (Cu), Silver (Ag), Gold (Au), and some elements with atomic numbers above 40.
Working Example
Let’s take, for example, Lithium with an atomic number of 3.
Take a look at the two configurations below.
• Configuration A shows a fully occupied 1s orbital and a half-occupied 2s orbital.
• Configuration B shows a half-occupied 1s orbital and a fully occupied 2s orbital.
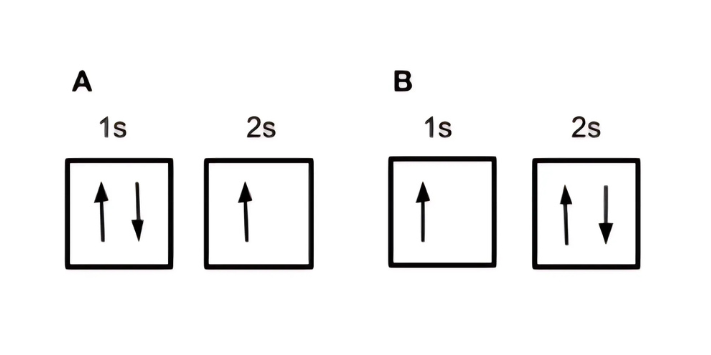
Among these, which do you think is the electron configuration of lithium?
Based on Aufbau’s Principle, the first two electrons should occupy the 1s orbital, and the last electron should fill the 2s orbital. In this case, configuration A represents the accurate orbital diagram for lithium. Following the process, the electron configuration for lithium will be 1s² 2s¹.
Orbital Capacities
Each type of atomic orbital (s, p, d, and f) can hold different numbers of electrons:
s-orbital → 1 orbital → holds 2 electrons max
The s subshell contains one orbital that holds a maximum of two electrons. For example, the 1s orbital in hydrogen (H) contains one electron, while helium (He) fills it completely with two electrons.
p-orbital → 3 orbitals → holds 6 electrons max
The p subshell contains three orbitals that hold a maximum of six electrons. For example, oxygen (O) has four electrons occupying the 2p sublevel.
d-orbital → 5 orbitals → holds 10 electrons max
The d subshell contains five orbitals that hold a maximum of ten electrons. For example, iron (Fe) has six electrons in the 3d sublevel.
f-orbital → 7 orbitals → holds 14 electrons max
The f subshell contains seven orbitals that hold a maximum of 14 electrons.
But the next question is, how do we fill orbitals if they are of the same energy?
Hund’s Rule
Hund’s Rule states that when filling orbitals with the same energy, known as degenerate orbitals, electrons will initially occupy these orbitals one at a time before any pairing happens. This helps minimize repulsion between electrons and keeps the atom at a stable, lower energy state.
Here are two key points to remember:
• Maximize unpaired electrons. Within a subshell, electrons will occupy each empty orbital first before sharing.
• Same spin alignment. The unpaired electrons will have parallel upward spins (↑↑↑), keeping their arrangement stable in line with quantum mechanics.
Atoms in their ground state tend to have as many unpaired electrons as possible. You can think of it like magnets, just as like poles repel each other, negatively charged electrons spread out to different orbitals before pairing up to maintain the lowest energy configuration and ensure stability.
Working Example
For example, consider the electron configuration of nitrogen (N), which has an atomic number of 7 and an atomic configuration of 1s2 2s2 2p3.

As shown in the illustrated configuration, the 1s and 2s orbitals are fully occupied. Meanwhile, in the 2p subshell, the remaining three electrons each occupy separate orbitals first, rather than pairing up. This follows Hund’s Rule, which favors maximum unpaired electrons in degenerate orbitals.
It’s also important to note that elemental nitrogen is most commonly found in nature as molecular nitrogen (N₂). In this form, it requires molecular orbitals, which are different from the atomic orbitals shown in the example above.
Pauli Exclusion Principle
Pauli's Exclusion Principle states that no two electrons in an atom can have the same four electronic quantum numbers.
In simple terms:
• An orbital can only be occupied by a maximum of two electrons.
• The electrons within the same orbital must have opposite spins—one spins up, the other spins down (↑↓).
Just think of each orbital as a bus seat where only two passengers (electrons) can sit at a time. But the catch is, they can’t sit in the same direction. One must face forward (↑), while the other must face backward (↓). If they both faced the same way, they'd bump into each other, just like two electrons in the same orbital can't have the same spin.
Here’s a visual representation of how electrons are arranged in the same orbital to understand the concept better.


.png)








.jpg)





.png)



%20-%20Thumbnail.png)
.png)
